Lipocine Inc. (NASDAQ: LPCN)
Lipocine Inc. (LPCN), a specialty pharmaceutical company yesterday announced that the FDA accepted its filing for a new drug application for LPCN 1021. LPCN 1021 is used as an oral testosterone replacement therapy for patients with low testosterone.
Lipocine Inc. Ceo Comments
“FDA acceptance of the NDA for LPCN 1021 is a significant milestone for both Lipocine and the millions of patients that could potentially benefit from an oral testosterone replacement therapy option,” said Dr. Mahesh Patel, Chairman, President and CEO of Lipocine Inc. “We will continue to work with the FDA as they complete their review.” Globe Newswire
LPCN Technical Analysis
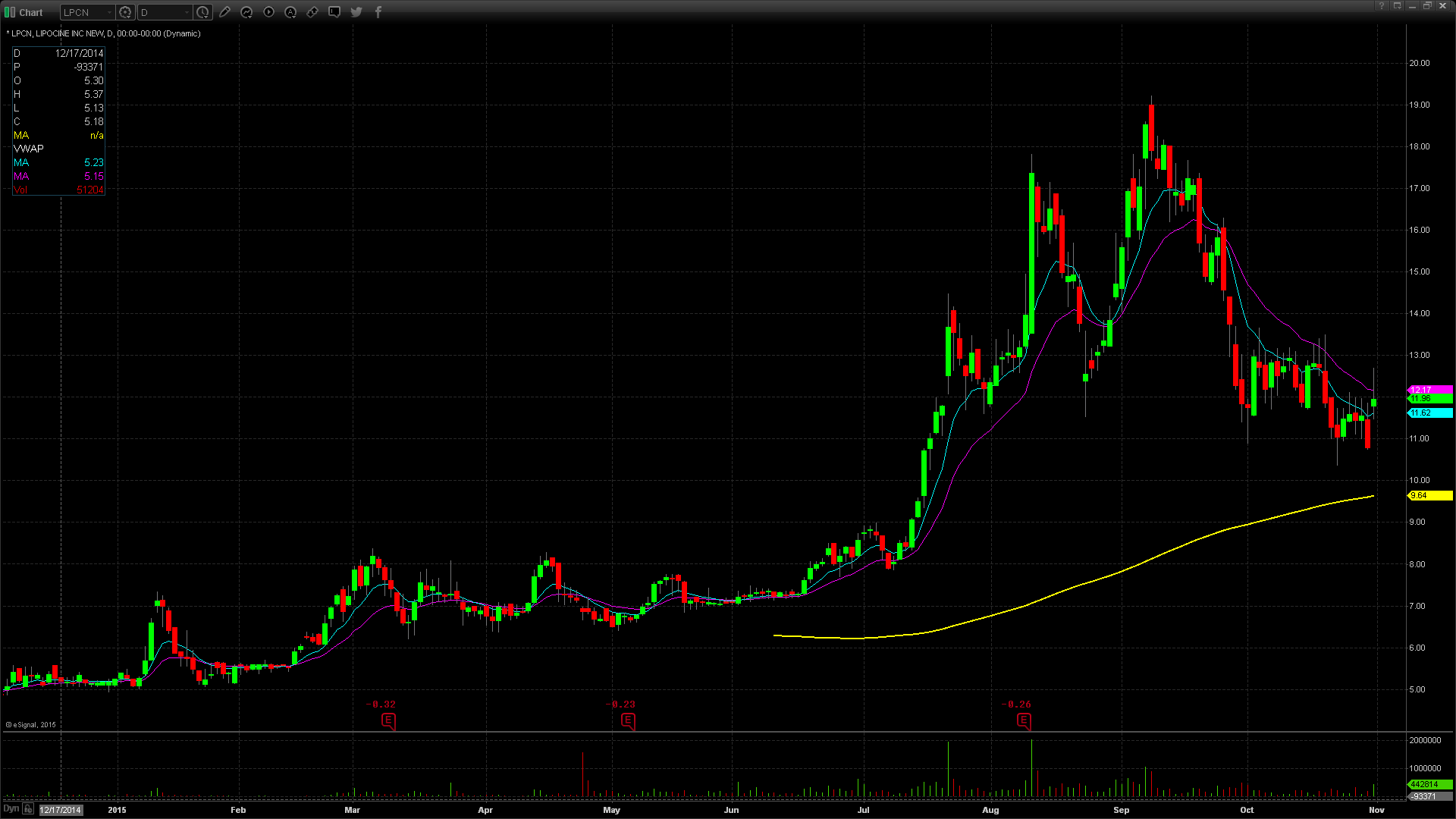
LPCN gapped up in price on Friday to $11.77, up from the prior day’s close of $10.77, which is a 9% increase in price based on favorable news. Taking a look at the daily chart, we can see the last time the stock traded above this price level was on October 20th, when it closed at $11.85. Taking a further look back on the chart, we can see the stock has been in an overall downtrend dating back to September 8th when the stock was trading at $18.54. More recently, LPCN has put in a mini triple top around the $12.85- $12.95 price level on October 2nd, 12th, and 16th. I would be looking for that price level to be the resistance for this current gap up spike. LPCN does have a low float of 12.77 million shares and is trading at the normal daily trading volume. The stock did reach pre market highs of $13 but it gave back $1.23 at the open, or equivalent to 9%. For trading purposes, my entry point would have been $11.85 looking for a run up to the $12.90 area. My stop loss would have been $11.65 fearing anything below that and the stock would start to fill in the gap up.
Company Profile
Lipocine Inc., incorporated on October 13, 2011, is a specialty pharmaceutical company. The Company is focused on applying its oral drug delivery technology for the development of pharmaceutical products in the area of men’s and women’s health. The company’s primary development programs are based on oral delivery solutions for bioavailable drugs. The company has a portfolio of product candidates designed to produce pharmacokinetic characteristics and facilitate lower dosing requirements, bypass first-pass metabolism, reduce side effects, and eliminate gastrointestinal interactions that limit bioavailability. The Company’s lead product candidate, LPCN 1021, is an oral testosterone replacement therapy (TRT), designed for twice-a-day dosing and is in Phase III testing. Additional pipeline candidates include LPCN 1111, an oral testosterone therapy product targeted for once daily dosing, which is in Phase II testing, and LPCN 1107, an oral therapy for the prevention of preterm birth, which is in Phase I testing. These products are based on its Lip’ral promicellar drug delivery technology platform. Lip’ral promicellar technology is a technology based on lipidic compositions which form an optimal dispersed phase in the gastrointestinal environment for improved absorption of insoluble drugs. Reuters