Foamix Pharmaceuticals Ltd. (NASDAQ: FOMX)
Foamix Pharmaceuticals Ltd. (FOMX), a clinical stage specialty pharmaceutical company yesterday announced positive top line results from their Phase II study for FDX104. FDX104 is a topical foam used for the treatment of rashes in patients getting targeted antibody treatments for colon, head and neck cancers.
Foamix Pharmaceuticals Ltd. CEO Comments
“There is a significant unmet need for a safe and effective treatment for EGFRI-induced rash, and we are pleased with the results of this clinical study,” stated Dov Tamarkin, Ph.D., Foamix’s CEO. “FDX104 has the potential to improve patients’ quality of life and help maintain patients on their optimal anti-cancer treatment. We are dedicated to developing best-in-class medicines that can have a positive impact on patients’ lives.” Globe Newswire
FOMX Technical Analysis
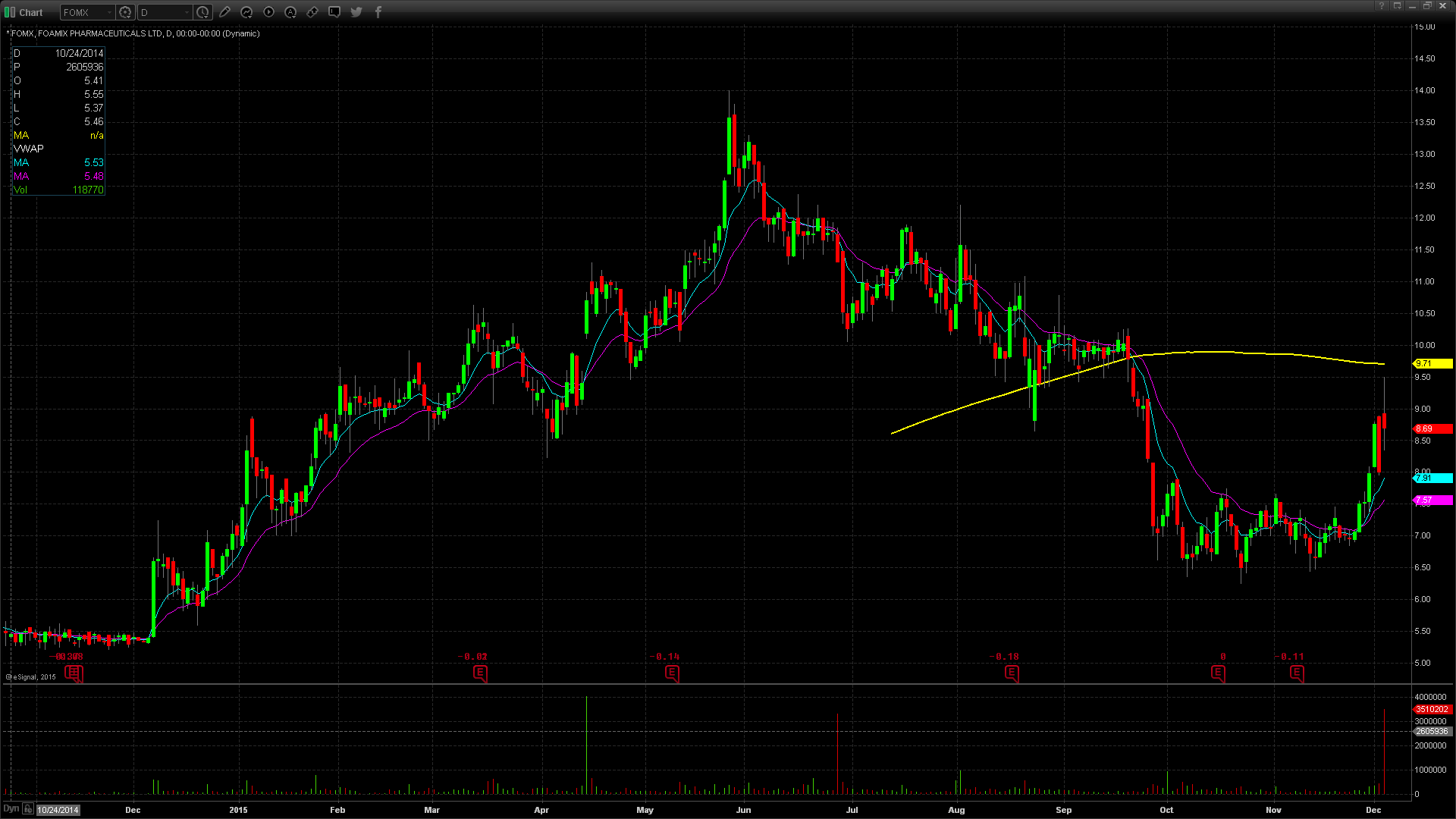
FOMX gapped up in price yesterday to $8.93, up from the prior day’s close of $8.00, which is a 12% increase on the favorable news. Taking a look at the daily chart, we can see the last time FOMX traded at this price level was on September 24th, when it traded at highs of $9.05. Taking a further look back on the daily chart, we can see that FOMX has been on an overall decline dating back to May 27th when it traded at its 52 week high price of $14. More recently, FOMX has been rebounding off of lows of $6.85 reached on November 23rd. The next resistance level would be $10 which was last reached on September 18th. FOMX does have a low float of 20.84 million shares and was trading over 6 times the normal daily trading volume. FOMX did reach pre market highs of $9.50, but gave back $0.57 at the open, or equivalent to 6%. For trading purposes, my entry point would have been $9.00 looking for a run to $9.50. My stop loss would have been $8.85, fearing anything below that and the stock would start to fill in the gap up.
Company Profile
Foamix Pharmaceuticals Ltd., a clinical-stage specialty pharmaceutical company, develops and commercializes foam-based formulations for the treatment of acne, impetigo, and other skin conditions in the United States, Germany, and Israel. Its lead product candidates include FMX101, a novel topical foam formulation which has completed a dose-ranging Phase II clinical trial for the treatment of moderate-to-severe acne; and FMX102 that has completed a Phase II clinical trial for the treatment of impetigo caused by methicillin-resistant staphylococcus aureus. The company is also developing FMX103, a version of FMX101 to treat rosacea; and FDX104 to treat chemotherapy-induced rashes, as well as early-stage stable foam formulations of various drugs for the treatment of dermatological indications, including atopic dermatitis, psoriasis, genital warts and actinic keratoses, and herpes and fungal infections. It has development and license agreements with Bayer HealthCare AG, Merz Pharmaceuticals, LLC, and Actavis plc. The company was founded in 2003 and is headquartered in Rehovot, Israel. Yahoo Finance