Intra-Cellular Therapies, Inc. (NASDAQ: ITCI)
Intra-Cellular Therapies, Inc. (ITCI), a biopharmaceutical company, announced yesterday positive top line results from the first Phase 3 Trial of their drug ITI-007. ITI-007 is used in the treatment of patients with schizophrenia. The study showed the drug had the desired antipsychotic effect with statistical superiority over the placebo.
Intra-Cellular Therapies, Inc. CEO’s Comments
“We are very encouraged by the positive results of our first Phase 3 trial. These data confirm the findings from our previous placebo- and risperidone active-controlled, randomized Phase 2 trial. The antipsychotic effect of 60 mg is confirmed and shows itself to be well-tolerated along with a safety profile similar to placebo,” said Dr. Sharon Mates, Chairman and CEO of Intra-Cellular Therapies. “Patients deserve a treatment choice which gives them symptom relief without the associated movement disorders, metabolic disturbances or cardiovascular effects observed with many antipsychotics. We are excited about our progress towards delivering a novel treatment option for patients.” Globe Newswire
Medical Expert Comments :
“ITI-007 demonstrated efficacy in the treatment of patients with schizophrenia,” said Dr. Carol Tamminga, Professor and Chairman, Department of Psychiatry, University of Texas Southwestern Medical School, and the Lou and Ellen McGinley Distinguished Chair in Psychiatric Research. “To have achieved efficacy at lower dopamine receptor occupancy levels more commonly associated with clozapine, arguably the most efficacious antipsychotic, while maintaining a placebo-like safety and tolerability profile is a remarkable advance in the development of drugs for schizophrenia.” Globe Newswire
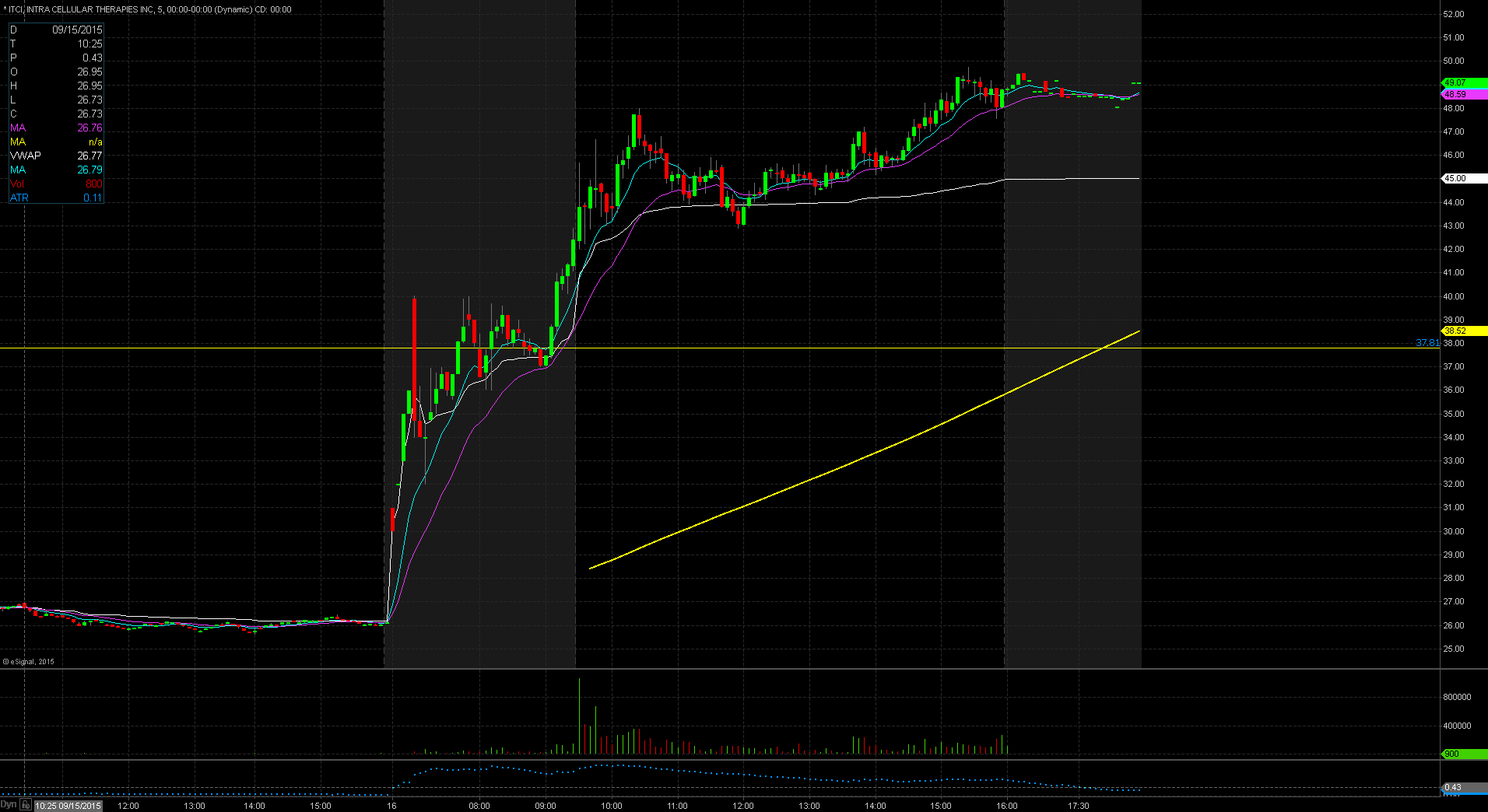
ITCI Technical Analysis
ITCI gapped up in price yesterday to $42.40 up from the prior day’s close of $26.11 which is a 62% increase in price based on favorable news. Taking a look at the daily chart we can see that we are in unchartered territory for the stock as its previous 52 week high was $37.8, reached on July 15. The stock does have a low float of 21.78 million shares and is trading on heavy volume today over 16 times normal. Both of these factors make the stock a prime candidate for the gap and go strategy. The stock did reach pre market highs of $42.90. For trading purposes, my entry point would be $42.50 with a possible add on at pre market highs of $42.90. My stop loss would be $42.25 fearing anything below that and the stock will start to fill in the gap up.
Company Profile
Intra-Cellular Therapies, Inc., a biopharmaceutical company, discovers and develops small molecule drugs for the treatment of neuropsychiatric and neurologic disorders within the central nervous system (CNS). Its lead drug candidate is ITI-007, which is in phase III clinical development for the treatment of schizophrenia, behavioral disturbances in dementia, bipolar disorder, depression, and other neuropsychiatric and neurological disorders. The company also develops ITI-002 program that is in phase 1 development for the treatment of central nervous system, cardiovascular, and other disorders. Intra-Cellular Therapies, Inc. has a license and collaboration agreement with Takeda Pharmaceutical Company Limited to research, develop, and commercialize its proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the prevention and treatment of human diseases. The company is headquartered in New York, New York. Yahoo Finance