Exelixis, Inc. (NASDAQ: EXEL)
Exelixis, Inc. (EXEL), a biopharmaceutical company made an announcement yesterday stating positive results from Phase 3 trial of Cobimetinib with Vemurafenib in Patients with BRAF V600 mutation of advanced melanoma. The study showed statistically significant increase in survival rate in patients receiving Cobimetinib.
Exelixis, Inc. CEO Comments
“The positive effect of the combination of cobimetinib and vemurafenib on overall survival is a major step forward for patients with advanced BRAF V600 mutation-positive melanoma in search of new treatment options,” said Michael M. Morrissey, Ph.D., president and chief executive officer of Exelixis. “We continue to work with our partners at Genentech in preparation for the potential U.S. approval and launch of cobimetinib to deliver this important potential treatment option to patients, physicians and caregivers in the United States as quickly as possible.” Business Wire
EXEL Technical Analysis
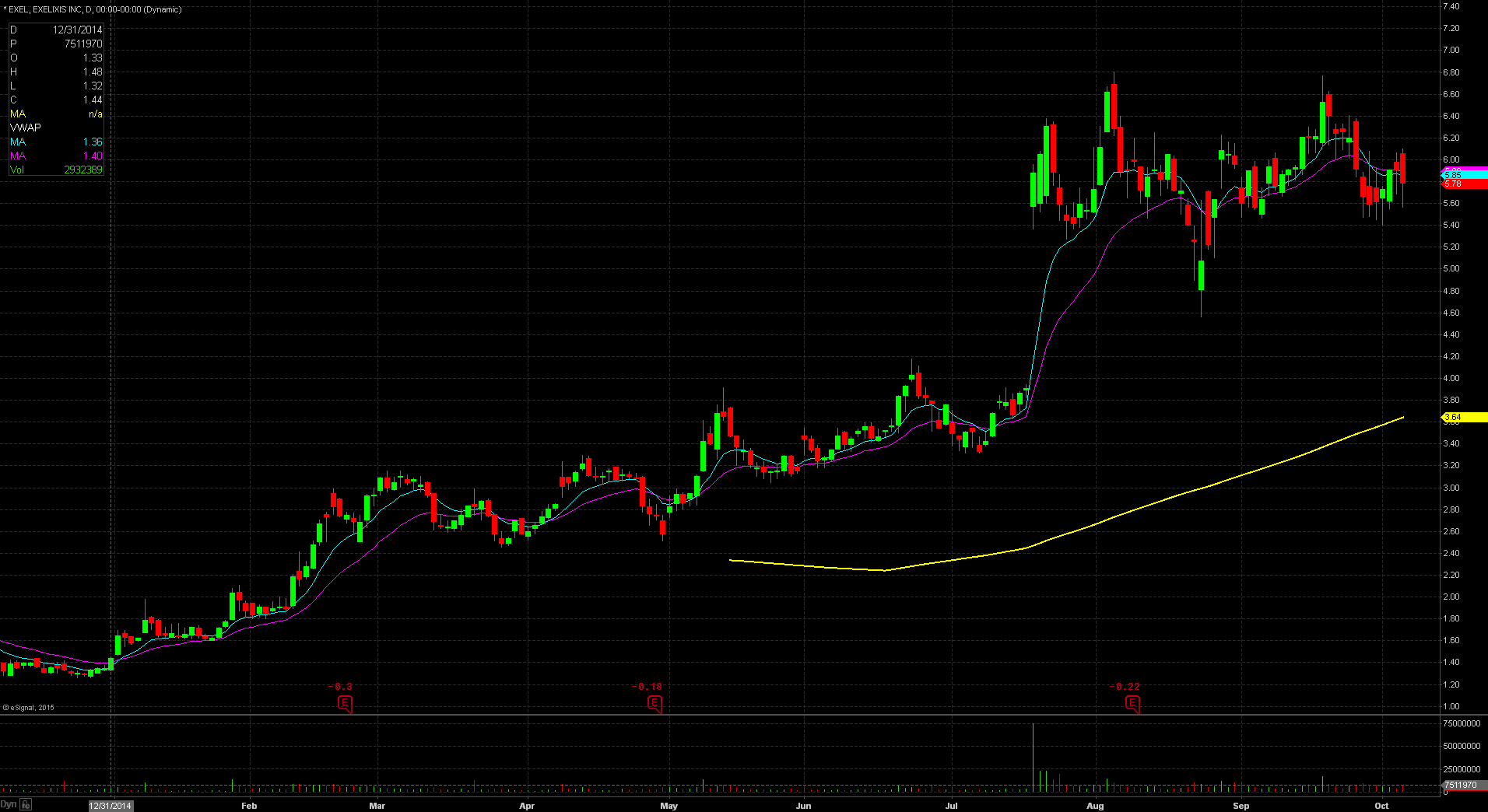
EXEL gapped up in price yesterday to $6.06 from the prior day’s close of $5.90 which is a 3% increase in price based on favorable news. Taking a look at the daily chart, we can see the last time the stock traded above this price level was on September 24th when the stock closed at $6.20. Taking a further look down the daily chart, we can see the stock popped twice on August 4th and September 18th to the $6.50 – $6.60 level. Both times the stock could not hold that level and fell down off that price. This should act as the first significant resistance level for the stock. The stock did reach pre market highs of $6.70, meaning that it gave back $0.64 at the open, or equivalent to 10%. For trading purposes, my entry point would have been $6.10 looking for a run to the $6.50 level. My stop loss would have been $6.00 fearing anything below that and the stock would start to fill in the gap up.
Company Profile
Exelixis, Inc., a biopharmaceutical company, develops and sells small molecule therapies for the treatment of cancer in the United States. The company offers COMETRIQ, an inhibitor of multiple receptor tyrosine kinases for the treatment of patients with progressive, metastatic medullary thyroid cancer. It is also evaluating cabozantinib in various development programs comprising approximately 45 clinical trials, including 2 ongoing phase 3 pivotal trials focusing on metastatic renal cell carcinoma (mRCC) and advanced hepatocellular carcinoma (HCC). In addition, the company develops cobimetinib, an inhibitor of MEK, which is in Phase 3 pivotal trial evaluating in combination with vemurafenib in previously untreated patients with unresectable locally advanced or metastatic melanoma harboring a BRAF V600 mutation. Exelixis, Inc. has collaborations with Genentech, Inc., GlaxoSmithKline, Bristol-Myers Squibb Company, Sanofi, Merck, and Daiichi Sankyo Company Limited for the development and commercialization of various compounds. The company was formerly known as Exelixis Pharmaceuticals, Inc. and changed its name to Exelixis, Inc. in February 2000. Exelixis, Inc. was founded in 1994 and is headquartered in South San Francisco, California. Yahoo Finance