Acadia Pharmaceuticals, Inc. (NASDAQ: ACAD)
On Monday, Acadia Pharmaceuticals, a biotech company focused on treatments for unmet central nervous system disorders, announced that the FDA has granted Priority Review of the company’s drug NUPLAZID, for the treatment of Parkinson’s disease psychosis. Under the Priority Review status, the FDA set a target action date for NUPLAZID of May 1, 2016.
ACAD Outlook
According to the National Parkinson Foundation, about one million people in the United States and from four to six million people worldwide suffer from Parkinson’s disease. An estimated 40 percent of these patients have Parkinson’s disease psychosis, which is characterized by hallucinations and delusions, a diminished quality of life, and significant caregiver burden. Business Wire. Many doctors and researchers see Parkinson’s patients doubling within five to ten years.
CEO Comments
“The FDA Priority Review designation underscores the potential for NUPLAZID to provide an important treatment to patients with Parkinson’s disease psychosis, a condition for which there is no FDA-approved therapy,” said Steve Davis, ACADIA’s President and Chief Executive Officer. “We look forward to working with the FDA during the review.” Yahoo Finance
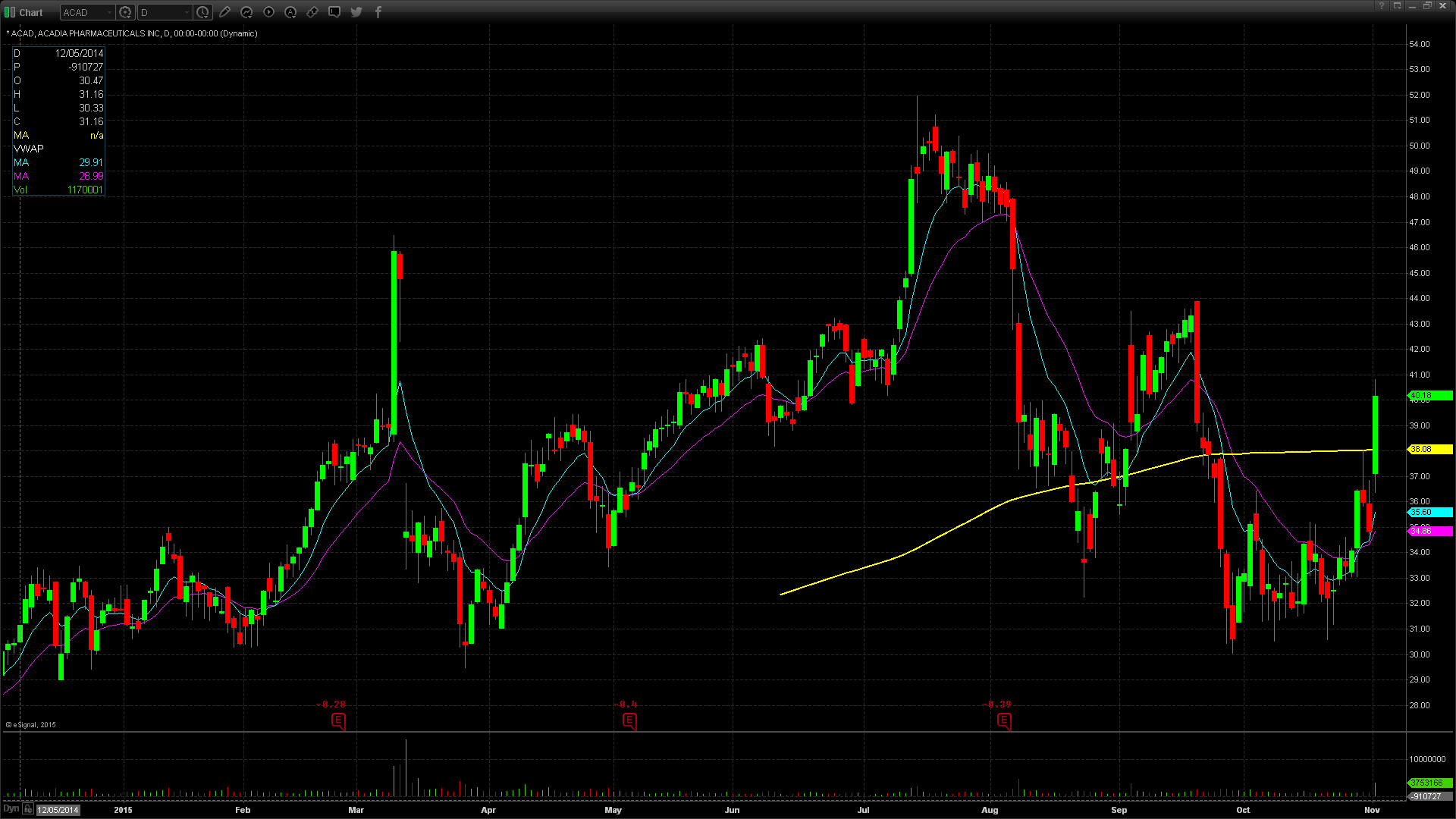
ACAD Technical Analysis
Monday’s FDA news catalyst fueled a 15.4% gain in Acadia stock, which closed the day up $5.36 at $40.18 on almost three times average daily volume. ACAD has generally traded in step with IBB, the closely watched biotech ETF. The stock hit all time highs of $51.99 in July before reversing with the rest of Biotechs to give back all 2015 gains by the end of September. ACAD spent most of October consolidating in a choppy range near the 2015 lows. Monday’s move out of this range to the upside was significant but it needs follow through. Big volume buying at the end of the day was a bullish sign, but ACAD must get through and hold $40.20 for the stock to be a conviction long. A move through Monday’s high of $40.84 could run to $42.
On the short side, a breach of $40, which has been a pivot level since May, shows weakness. A move below this level could lead to the next support at $39.60’s. Below that, bears are in control.
ACAD moved today on significant FDA news. Over the next several days, the experts will digest this news and drive the stock accordingly. ACAD reports financial results on Thursday, November 5 after the market close. Keep in mind that ACAD has 18.4% short float.
About Acadia Pharmaceuticals, Inc.
ACADIA Pharmaceuticals Inc. is a biopharmaceutical company. The Company is focused on the development and commercialization of medicines for the treatment of neurological and related central nervous system disorders. The Company’s drug candidate NUPLAZID (pimavanserin) is under development for the treatment of Parkinson’s disease psychosis, Alzheimer’s disease psychosis, Schizophrenia and Sleep disturbances. The Company is also conducting preclinical programs for alpha adrenergic agonists indicated for the treatment of chronic pain and muscarinic drugs for the treatment of glaucoma. NUPLAZID has an active ingredient pimavanserin, is a selective serotonin inverse agonist (SSIA) preferentially targeting 5-HT2A receptors that was discovered by the Company. The Company in collaboration with Allergan, has discovered and engaged in the development of small-molecule product candidates, such as alpha adrenergic agonists and muscarinic receptors. Google Finance