The past couple of weeks has seen a series of biotech companies plummet amidst poor news from federal regulators.
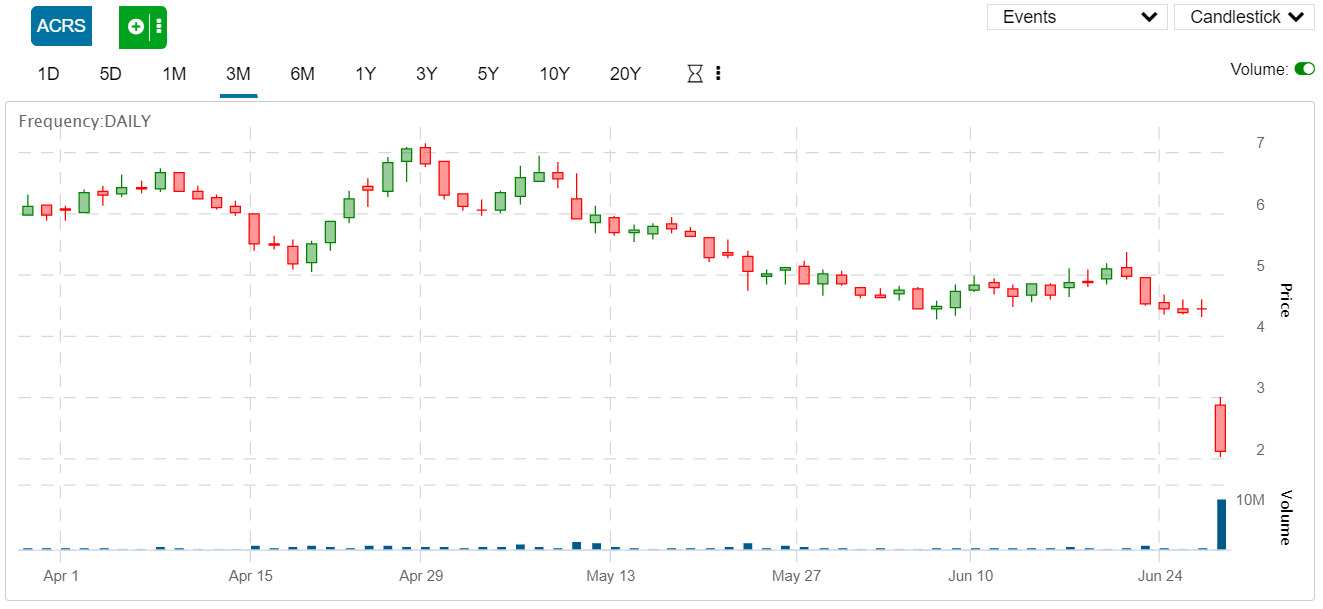
Specifically, shares of Aclaris Therapeutics (NASDAQ: ACRS) dropped by 52 percent today after the company announced that one of its most important drug candidates, ATI-502, failed to meet neither its primary or secondary endpoints in its phase 2 trial. Even worse, it turned out that their main drug candidate did worse then the placebo.
ATI-502, a topical treatment that’s targeted towards curing alopecia areata (also known as male pattern baldness) was largely ineffective according to the results of the trial. Evaluating two groups of patients, one applying ATI-502 and another without the compound, found that the placebo group had as much if not greater results than the group using ATI-502.
“We are surprised and extremely disappointed by the results of this Phase 2 trial,” said Dr. Neal Walker, President and Chief Executive Officer of Aclaris, in a press release. “This is disappointing not only for the company, but also for patients who are living with alopecia areata. We sincerely thank the patients and investigators who participated in this trial. We look forward to advancing our other development programs.”
Investors didn’t react well to the news that a placebo had outperformed the actual drug in question. These results also refute an earlier phase 2 trial announced 10 days ago, which showed that ATI-502 triggered around 15.3 hairs per square centimeter for women and 5.6 hairs per square centimeter for men. However, this trial wasn’t compared to anything else, like another compound or a placebo, so the results are difficult to quantify and compare.
Shares of Aclaris fell by 52 percent in response to today’s news. Like many other small biotech companies, stock prices tend to fall over time the longer the company goes without making a major breakthrough before shares spike up again.
However, Aclaris hasn’t been able to catch a break for a long time. Over the past 12 months, shares of the 90-million-dollar company have fallen by over 90 percent, declining from $20.5 per share down to just over $2.13 as of Thursday.
At this point, Aclaris will have to figure out how to regroup after the disappointing results and whether conducting more clinical studies at all will prove helpful in this regard. Regardless, it’s never good news when a biotech’s signature drug candidate gets outperformed by the placebo group.
Aclaris Therapeutics Company Profile
Aclaris Therapeutics Inc is a clinical-stage specialty pharmaceutical company which operates in United States. It focuses on identifying, developing and commercializing differentiated drugs to address significant unmet needs in dermatology.
The company holds a drug candidate, A-101 Topical Solution which is a proprietary high-concentration hydrogen peroxide topical solution which is being developed as a prescription treatment for seborrheic keratosis (SK) a common non-malignant skin tumor.
Its pipeline of drug candidates includes A-101 as a Topical Solution for treatment of SK, common warts; A-102 as a gel dosage form for treatment of SK and warts and A-201 and A-301 as an oral and topical formulation for treatment of autoimmune dermatologic respectively. – Warrior Trading News